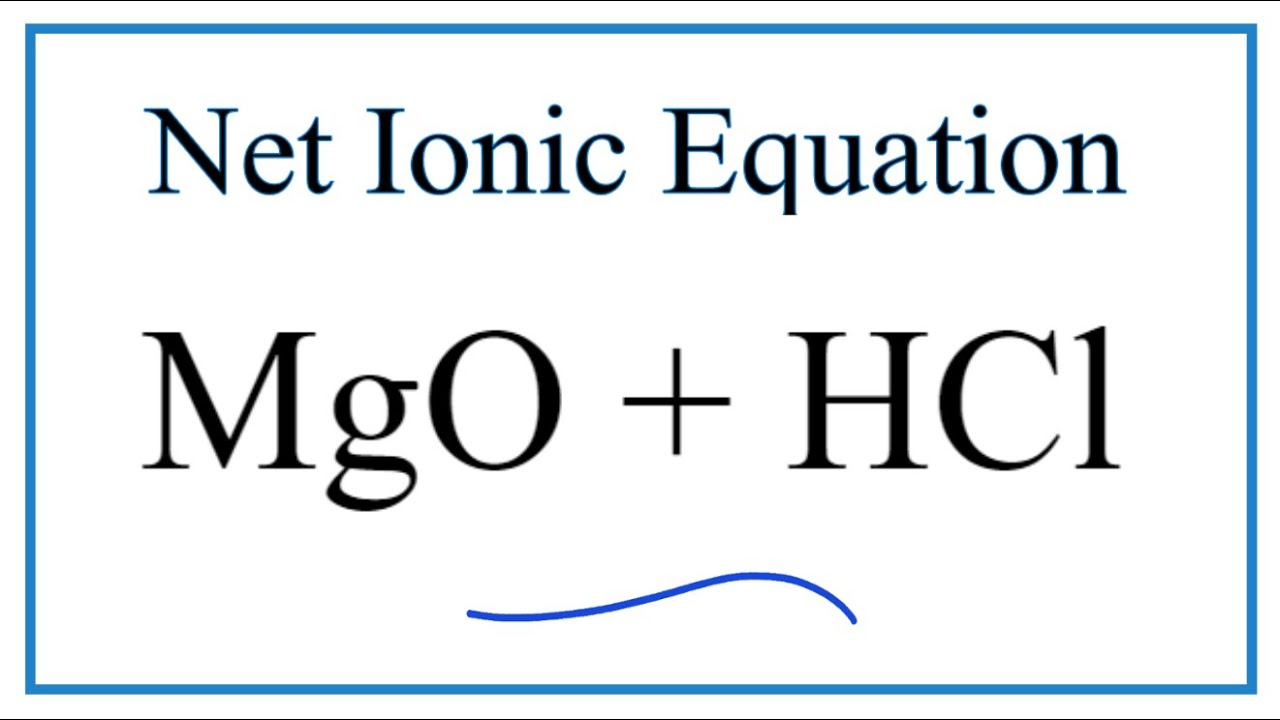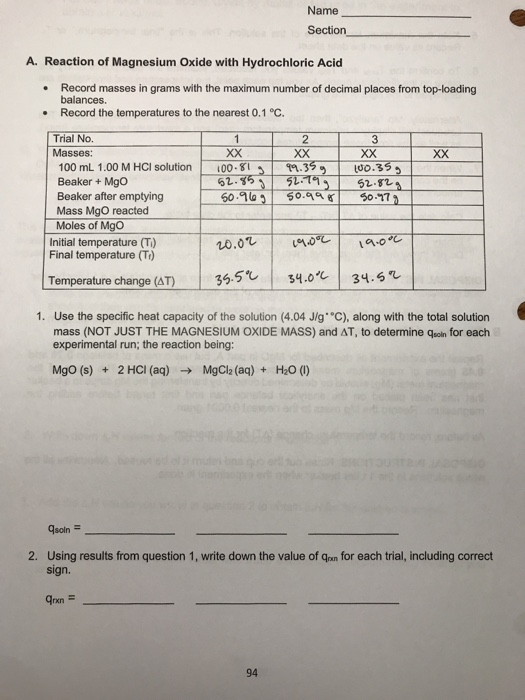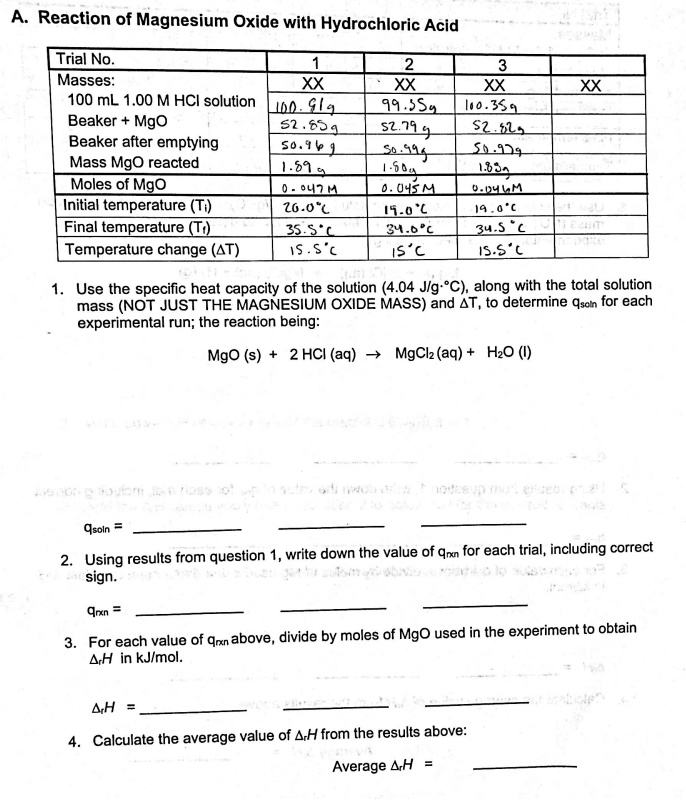
SOLVED: Reaction of Magnesium Oxide with Hydrochloric Acid Trial No Masses: XX XX 100 mL 00 M HCI solution Jno Ghs 91. 5S4 1o.31 Beaker Mgo 52 629 52 ,219 S2,6L) Beaker

SOLVED: 2. Magnesium oxide, MgO, is not very soluble in water, and is difficult to titrate directly. Its purity can be determined by use of a 'back titration' method. 4.06 g of

Introduction to Acids and Bases. Acid A substance that produces hydrogen ions, H + (aq), when it dissolves in water. Sour-tasting and good conductors. - ppt download
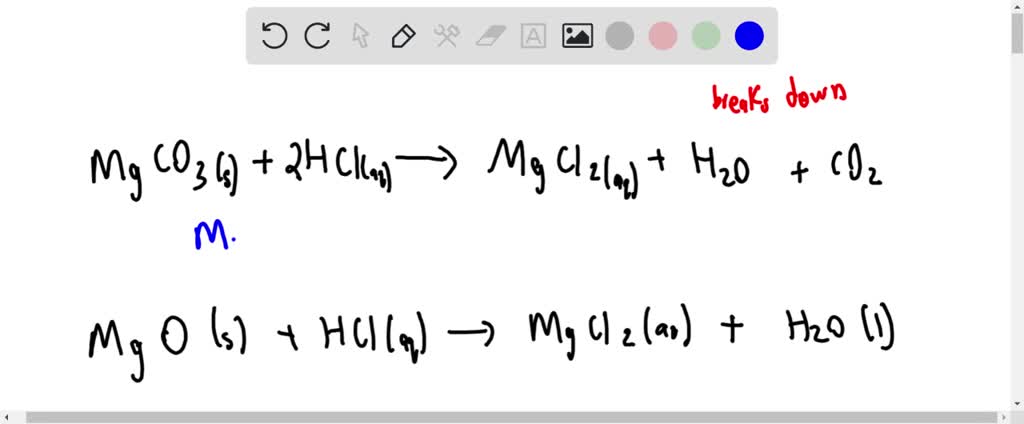
SOLVED:Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a net ionic equation for the reaction that occurs

Write the balanced chemical equations for the following reaction:Calcium hydroxide + Carbon dioxide → Calcium Carbonate + Water.

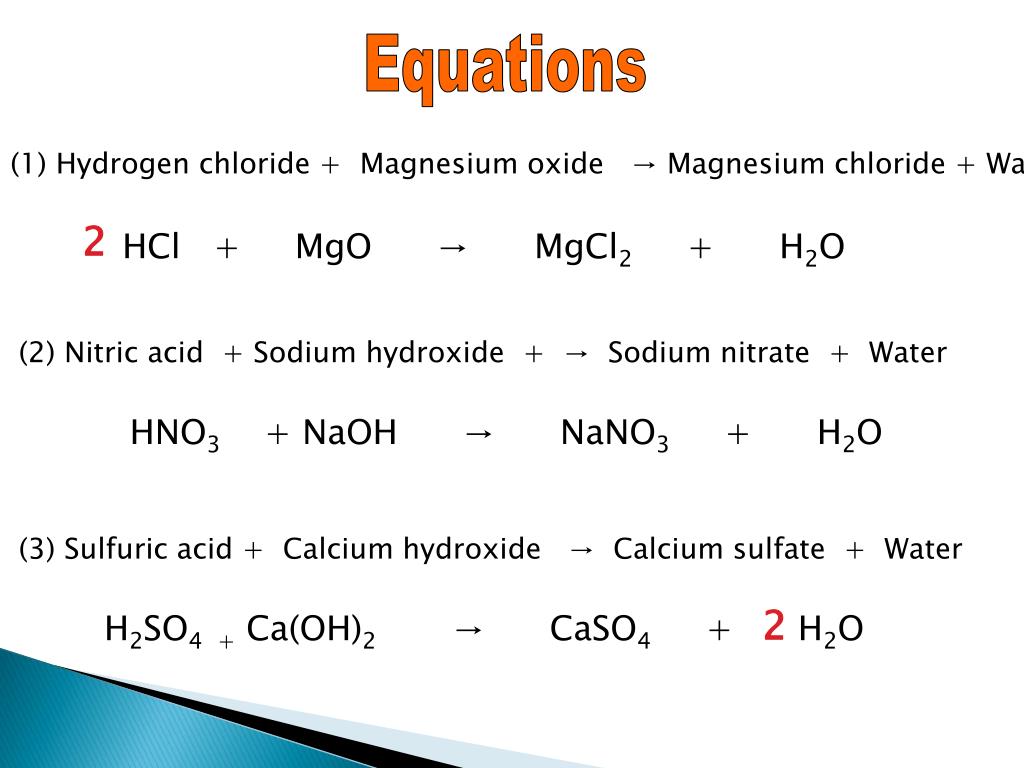
Ionic equations A chemical equation shows the number of atoms and molecules of the reactants and products. Also shows physical state of reactants and products. - ppt download