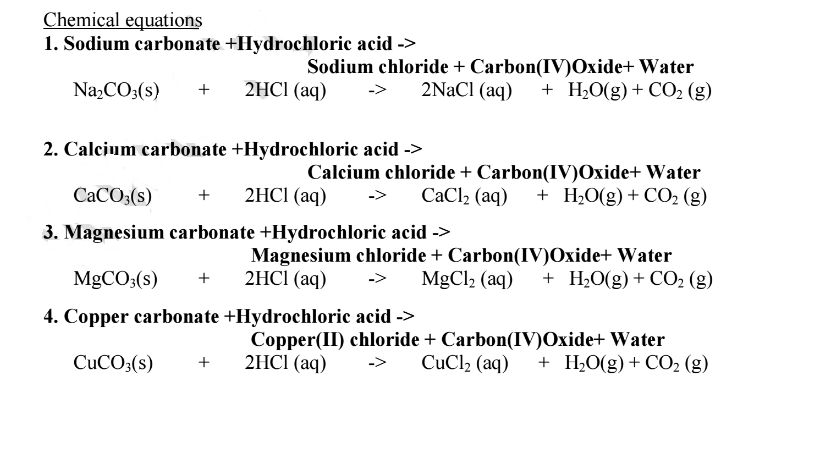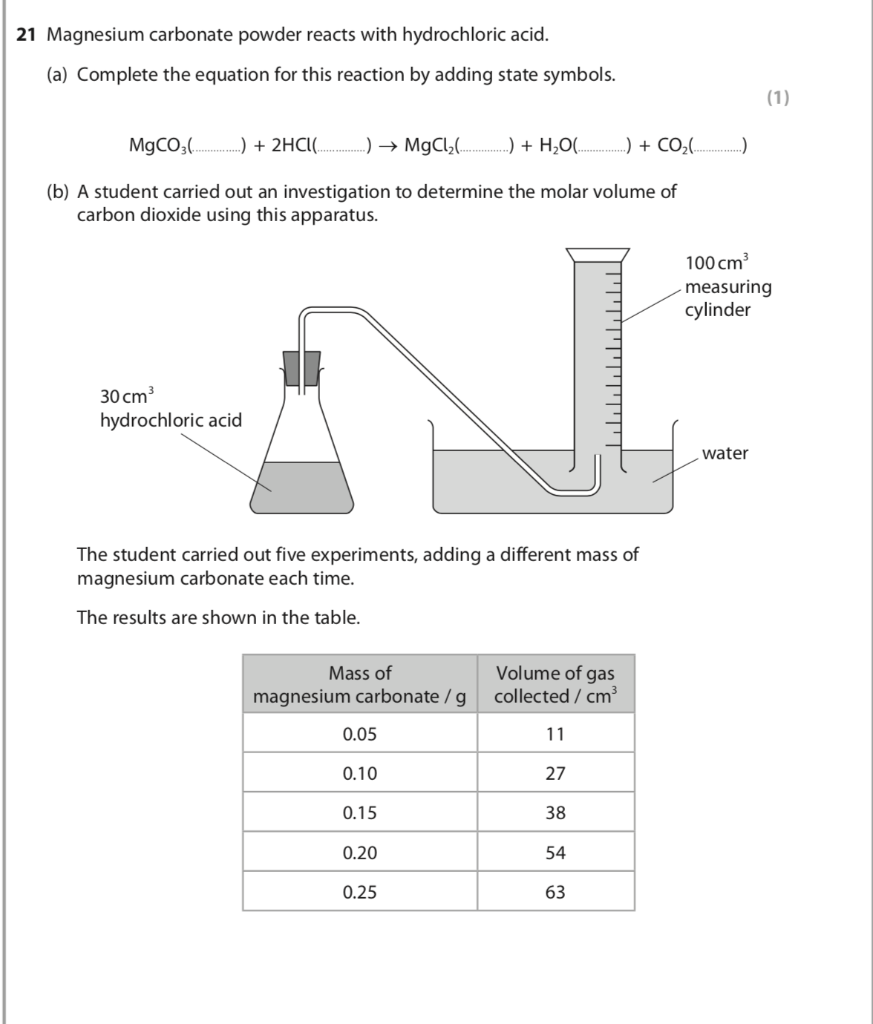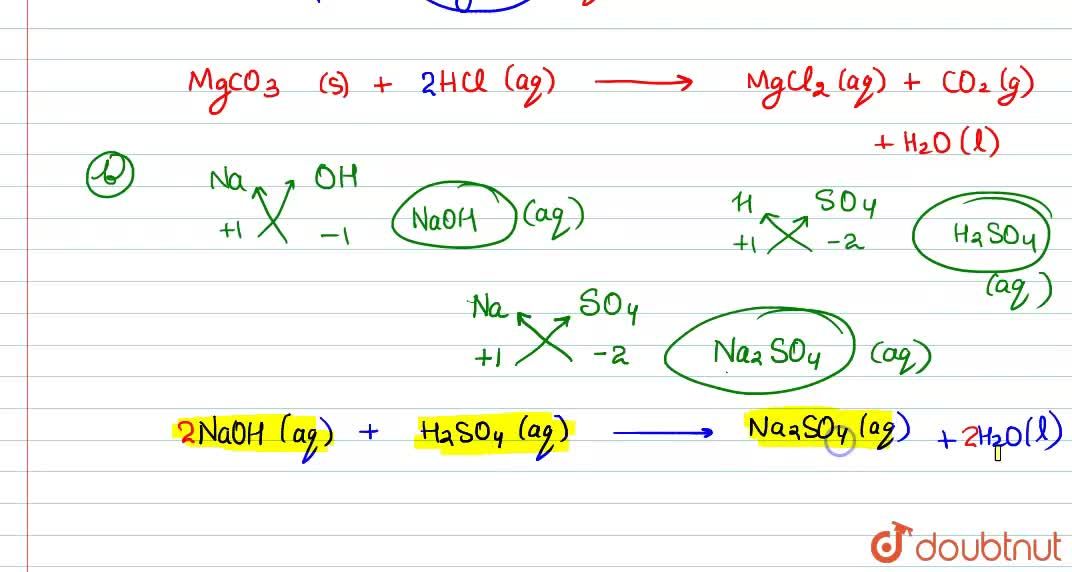
Write the balanced equations for the following reactions, and add the state symbols : (a) Magnesium carbonate reacts with hydrocloric acid to produce magnesium chloride, carbon dioxide and water. (b) Sodium hydroxide

balanced equation for the following a)megnesium carbonate reacts with HCL acid to produce megnesium - Brainly.in
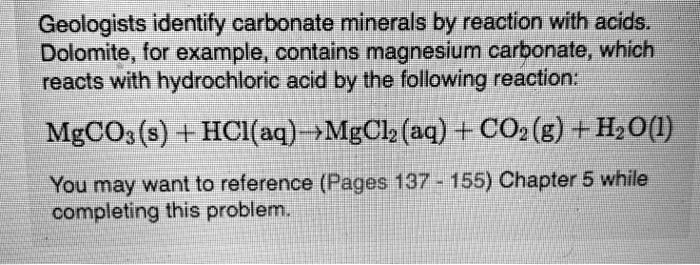
SOLVED: Geologists identify carbonate minerals by reaction with acids Dolomite , for example , contains magnesium carbonate , which reacts with hydrochloric acid by Ihe following reaction: MgCOs (s) + HCl(aq) ,MgClz (

Write the balanced equations for the following reactions, and add the state symbols : (a) Magnesium carbonate reacts with hydrocloric acid to produce magnesium chloride, carbon dioxide and water. (b) Sodium hydroxide

Calcium carbonate reacts with dilute hydrochloric acid according to the equation below - GCSE Science - Marked by Teachers.com